The Strength of an Acid or Base Depends on
Values of the pH of 010 M solutions of a number of common acids and bases are given in the table below. The chemical reaction involved in acid-base titration is known as neutralisation reaction.

Relating Acid Base Properties To The Periodic Table
The system is said to be in equilibrium when the concentrations of its components will not change over time because both forward and backward reactions are occurring at the same rate.

. Thats not necesarilly the case as it depends on the solution temperature and ionic strength of the solution besides slight hydrolysis of NaOH shifts pH down by about 002 unit. Not that it changes much -. In this type of titration the strength of a solution is determined by its complete precipitation with a standard solution of another substance.
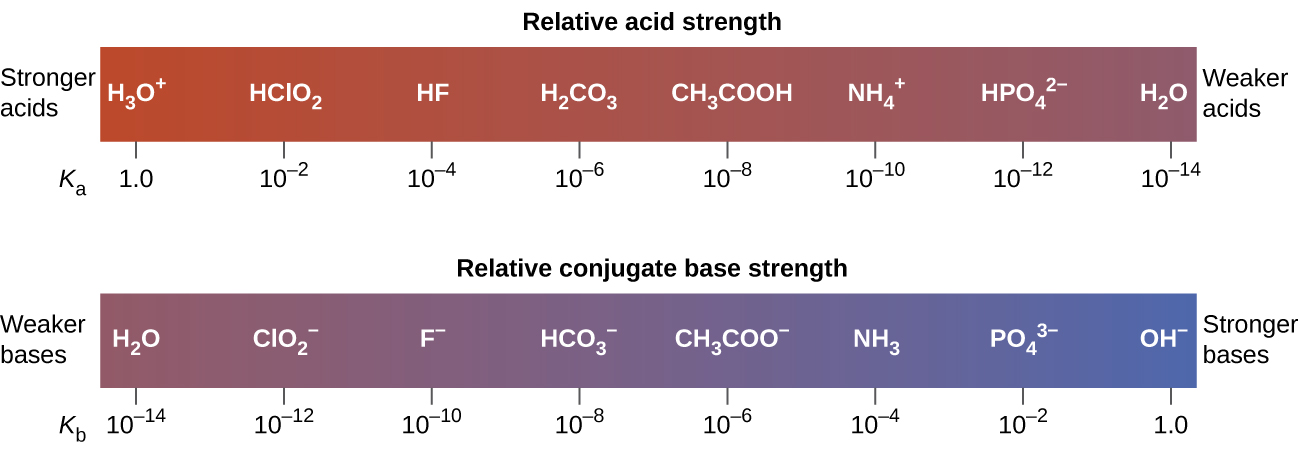
You can estimate the pH value of the equivalence point according to the following rule. The pH of a solution depends on the strength of the acid or base in the solution. In fact the hydrogensulphate ion is a relatively weak acid - similar in strength to the acids we have already discussed on this page.
Because the solvent system definition depends on the solute as well as on the solvent itself. An acidbase reaction is a chemical reaction that occurs between an acid and a base. The pH value of the solution obtained at the equivalence point depends on the relative concentration of acid and base.
It involves the combination of H 3 O ions with OH-ions to form water. This time you get an equilibrium. To the electrostatic and covalent contributions to the strength of the bonds that the acid and base will form.
Strong acid reacts with strong base to form an acidic solution pH 7. Equivalence point of strong acid titration is usually listed as exactly 700. Measurements of the pH of dilute solutions are therefore good indicators of the relative strengths of acids and bases.
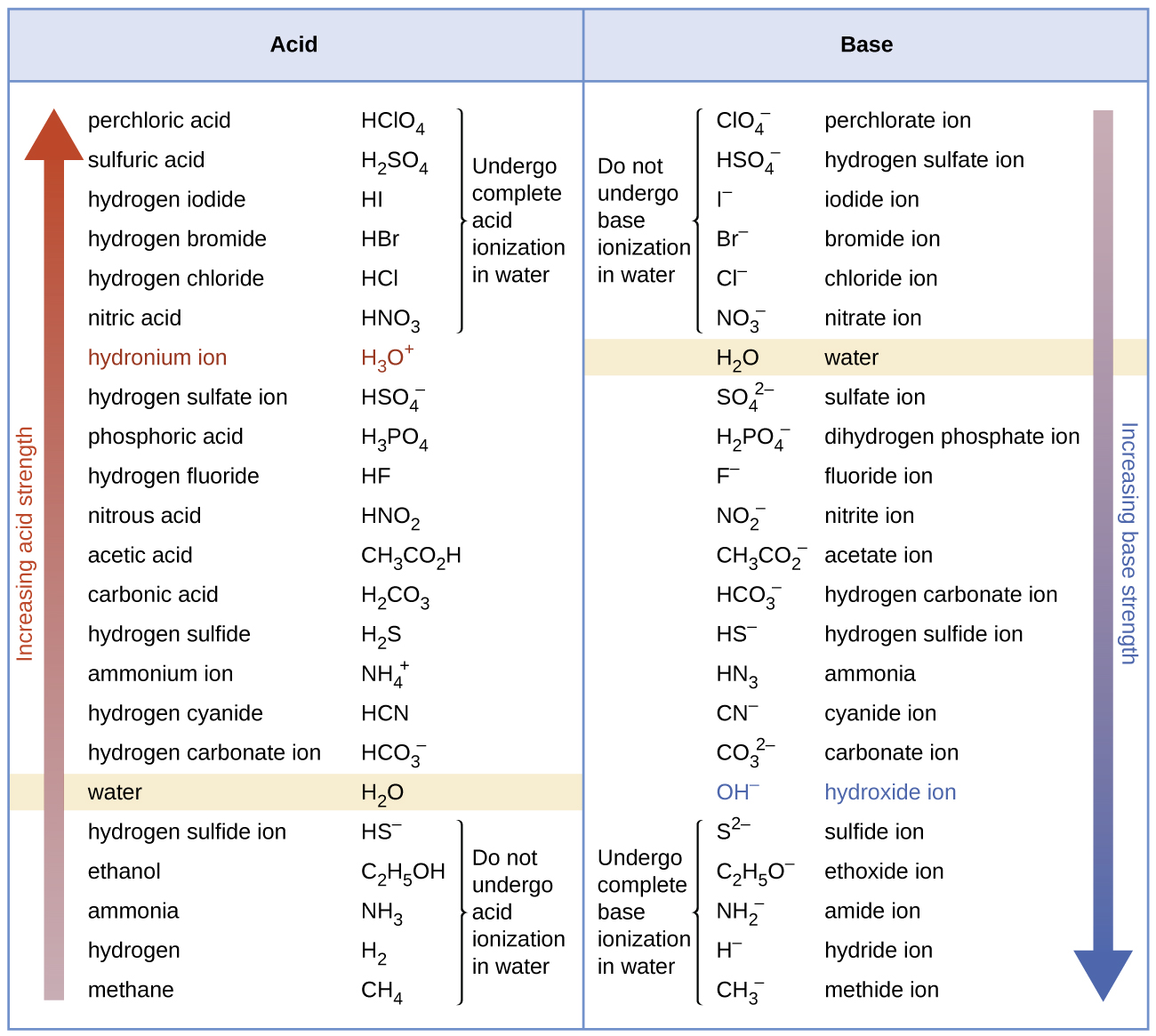
Strong acid reacts with weak base to form an acidic solution pH 7. HSO 4-aq H 2 Ol H 3 O aq SO 4 2-aq Sulphuric acid of course has all the reactions of a strong acid that you are familiar with from introductory chemistry courses. Known as dissociation in the context of acidbase reactionsThe chemical species HA is an acid that dissociates into A the conjugate base of the acid and a hydrogen ion H.

Https Www Slideshare Net Educationchemist Acids And Bases 2230135 Dissociation Molecules Equations
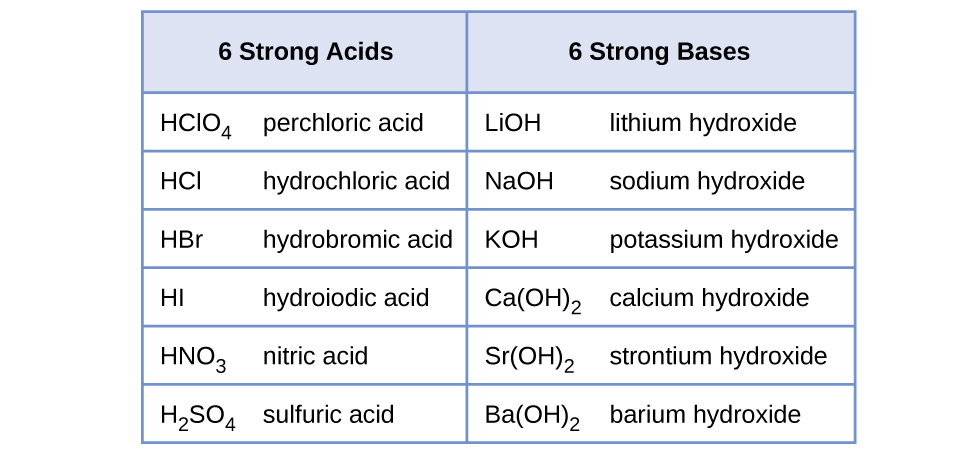
14 3 Relative Strengths Of Acids And Bases Chemistry
No comments for "The Strength of an Acid or Base Depends on"
Post a Comment